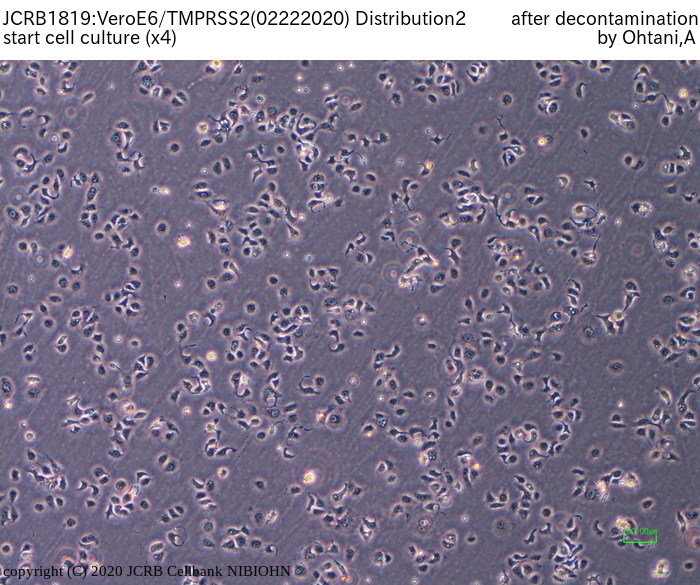
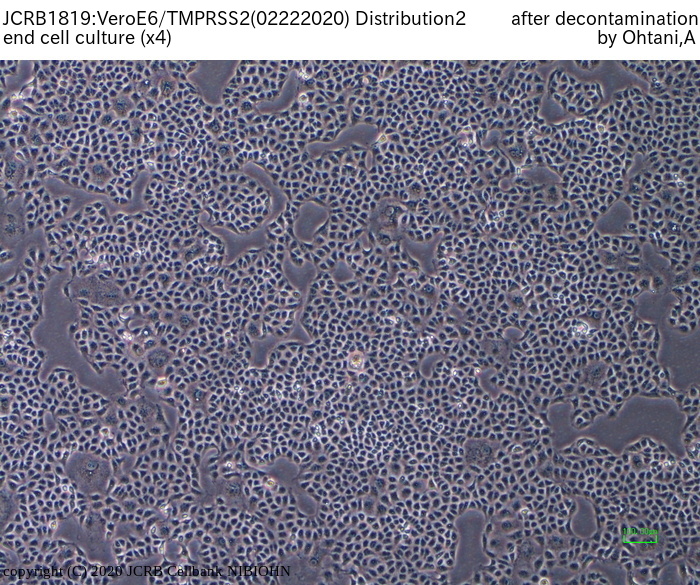
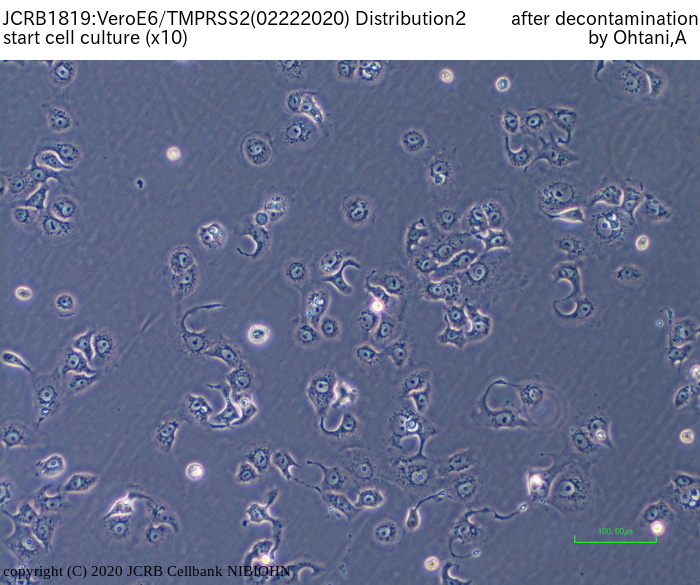
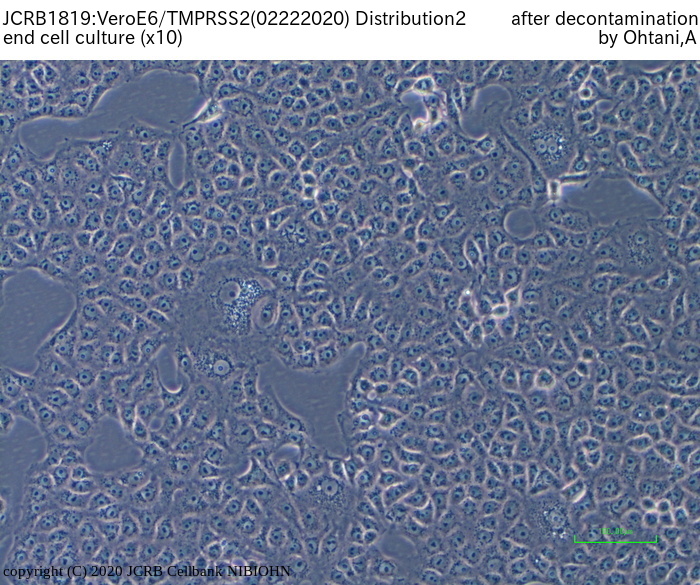
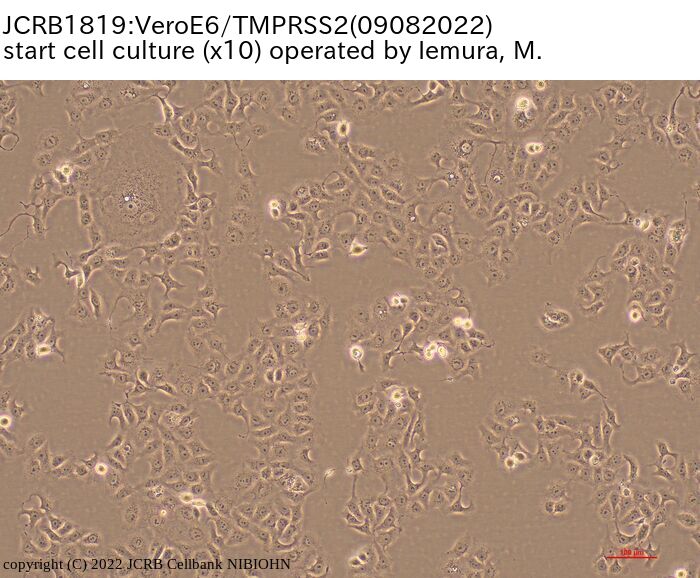
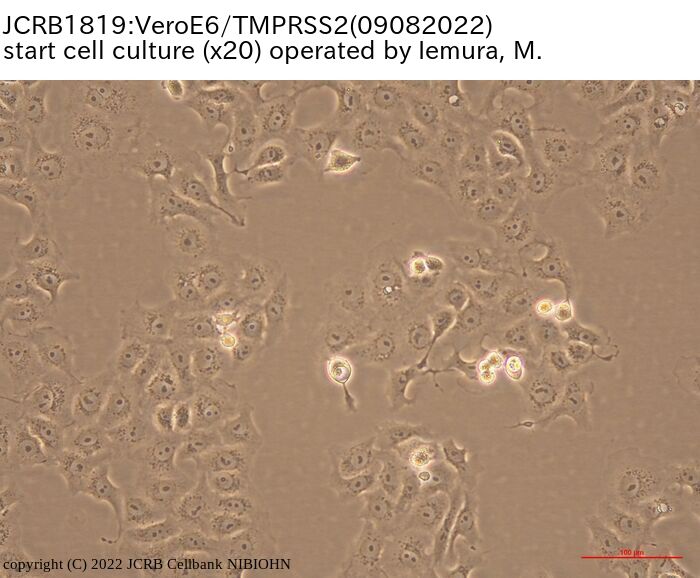
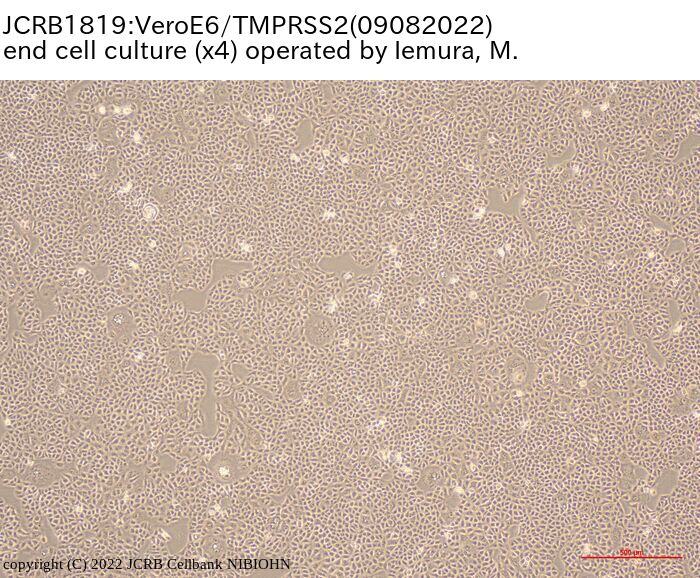
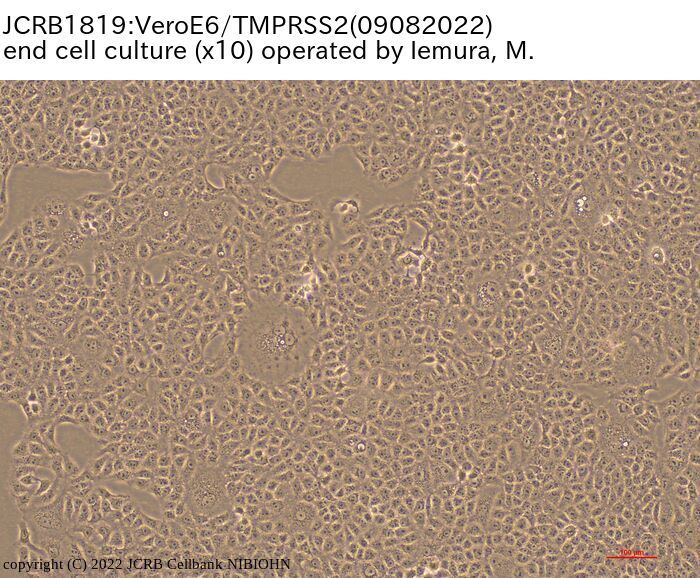
[On the commercial use of Vero/TMPRSS2]
If the Vero/TMPRSS2 is intended to use for commercial purposes such as vaccine production, the contract with the developer, National Institute of Infectious Disease will be required. So, please note that JCRB may disclose the purposes of usage to NIID when this cell line is requested by customer.
[本細胞のワクチン製造等の商業利用に関して]
Vero/TMPRSS2をワクチン製造等の商業利用目的で使用する場合には、樹立機関である国立感染症研究所との契約等が必要となります。このため、利用者から本細胞の分譲依頼があった場合、JCRBはその使用目的を国立感染症研究所に開示することがあることをご承知おきください。
Feb. 7th, 2020.
[Close]
(Feb 2020)
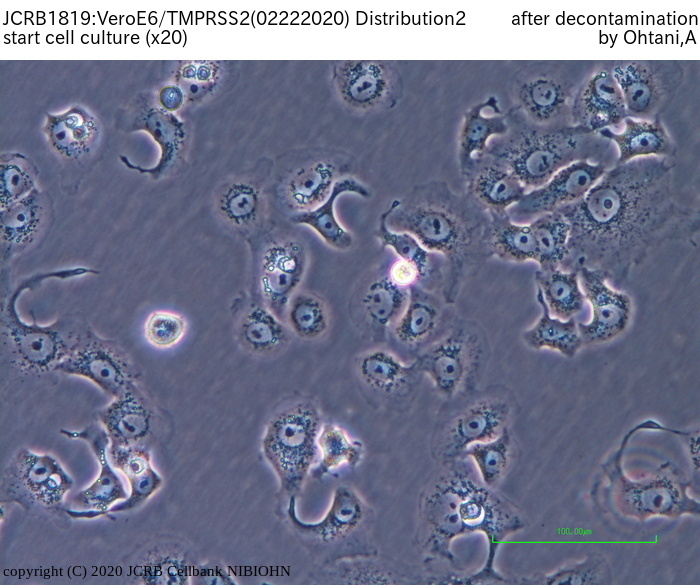
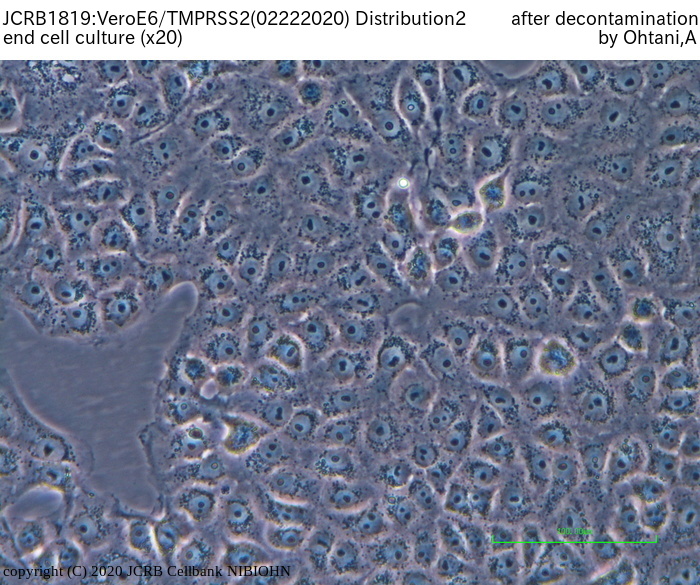
The deposited VeroE6/TMPRSS2 found to be contaminated by mycoplasmas.
JCRB treated the cells with anti-mycoplasmal reagents to remove the mycoplasmas, and current test for mycoplasma was negative (20 Feb. 2020). But JCRB can not judge that the removal of mycoplasma was successfully done, because there is a possibility that the mycoplasma may again become positive. So JCRB is currently maintaining the cells without antibiotics for additional 3 months, and plan the final test in May - June 2020.
So, please note that the test for mycoplasma contamination of this cell line has not finally been concluded at present.
(Append on 11-Mar-2020)
The lot which Myco-Alert(-)negative was ready. JCRB will continue culture without antibiotics to check this lot again after 3-months and then open for distribution.
However, considering the present situation, JCRB distributes the current lot if researchers need Myco-Alert(-) lot in full awareness of this state. Please contact JCRB directly.
(Append on 23-Apr-2020)
Currently, we carried out continuous culture for 60 days, and the negative has been confirmed by Myco-Alert, Nested-PCR, and DNA fluorescent staining. JCRB will continue culturing for 3 months as above, and will finally confirm that mycoplasma is not detected again.
(Append on 3-June-2020)
Final tests for mycoplasma after 3 months monitoring was negative (nested PCR, DAA fluorescent staining and MycoAlert tests). JCRB confirms that elimitation of mycoplasma from VeroE6/TMPRSS2 was successfully done.
(2月8日)現在緊急対応として増殖を進めている本細胞において、マイコプラズマが検出されました。検査は簡易検査(マイコアラート)で実施しております。今後の細胞分譲に関してはマイコプラズマ感染した細胞でも利用希望される方に提供を継続します。細胞バンクでは細胞のマイコプラズマ除染も開始しましたので、除染された細胞を御希望の方は提供まで3か月程度かかってしまいます事、予めご了承ください。
(3月11日追記)現在、マイコプラズマ除去処理し簡易検査(MycoAlert)により陰性確認したロットが作製できております。
通常はこのまま3か月間継続培養を行い、マイコプラズマが再検出されないことを確認してから分譲実施しますが、今回の細胞に関しては、このような状況であることをご承知の上、希望される方には分譲を実施させて頂きます。ご希望の場合は細胞バンクまでお申し込みのうえ、ご連絡ください。
(4月23日追記)現在、60日間の継続培養を実施し、簡易検査(MycoAlert)およびNesuted-PCR、DNA蛍光染色法により陰性が確認できています。このまま3か月間継続培養を行い、マイコプラズマが再検出されないことを最終確認します。今回の細胞に関しては、このような状況であることをご承知の上、希望される方には分譲を実施させて頂きます。ご希望の場合は細胞バンクまでお申し込みのうえ、ご連絡ください。
(6月3日追記)3か月間の継続培養後の最終的なマイコプラズマ検査にてもマイコプラズマ陰性を確認しました(Nested PCR, DNA染色, マイコアラート検査)。VeroE6/TMPRSS2細胞のマイコプラズマ汚染除去は確実に成功していると結論づけられました。
[Close]
VeroE6/TMPRSS2 is derived from African green monkey that is listed in CITES (Washington Trustee) Appendix II. Therefore the exportation of VeroE6/TMPRSS2 is regulated by CITES(*1).
In the exporation of this cell line, the CITES export permit from Japanese Government is required. The time required for obtaining CITES permission may take very long time (1-3 months). In addition, you are requested to get the CITES import permit from government of your country in the importation of CITES regulated items after we get the CITES export permit. Therefore the exportation of this cell line needs considarably long lead time.
In addition, popular courier such as FedEx, DHL, UPS will not handle the CITES regulated materials. Therefore the shipping costs will become very high.
If the customer agree with the above matters, we will respond the oversea customer's request for distribution considering of the importance of this cell line although JCRB website says this cell line as "Japan domestic use only".
(*1)Convention on International Trade in Endangered Species of Wild Fauna and Flora (Washington Trustee).
[Close]
[To customers in Europe about VeroE6/TMPRSS2]
The developer of VeroE6/TMPRSS2 has deposited VeroE6/TMPRSS2 to National Institute for Biological Standards and Control(NIBSC) in the United Kingdom.
Therefore researchers in Europe are recommended to obtain it from NIBSC.
Please contact NIBSC for any questions.
Email: cfar@nibsc.org
[Close]
| JCRB No. |
JCRB1819 |
Cell Name |
VeroE6/TMPRSS2 |
| Profile |
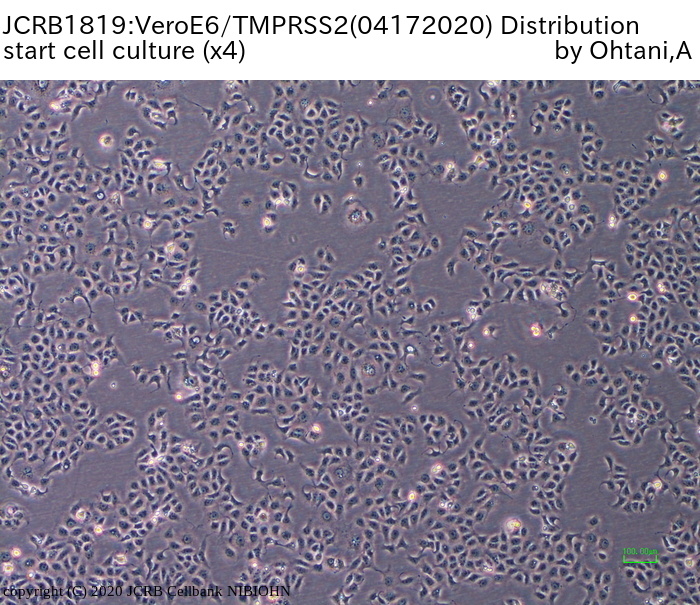
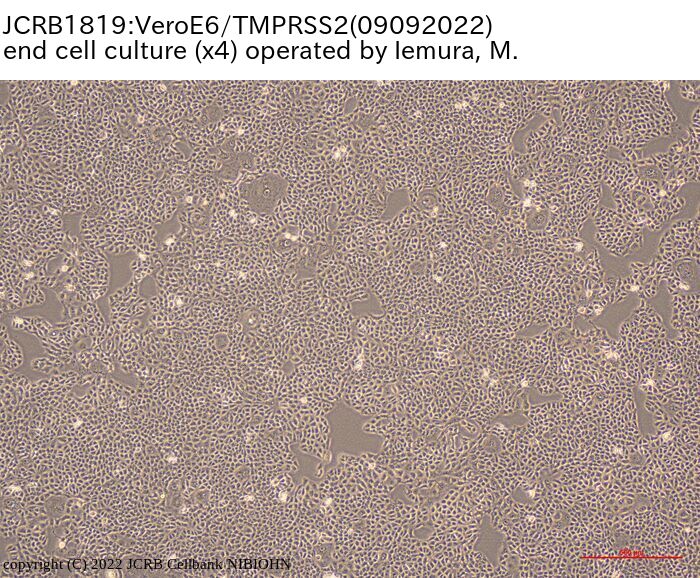
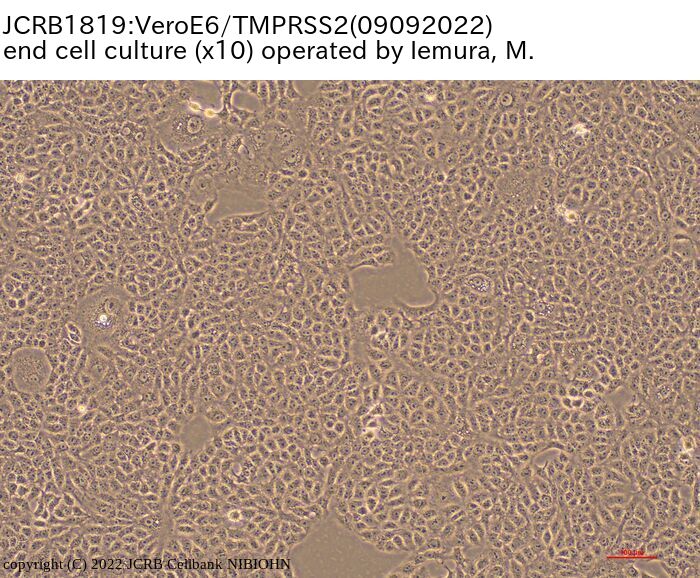
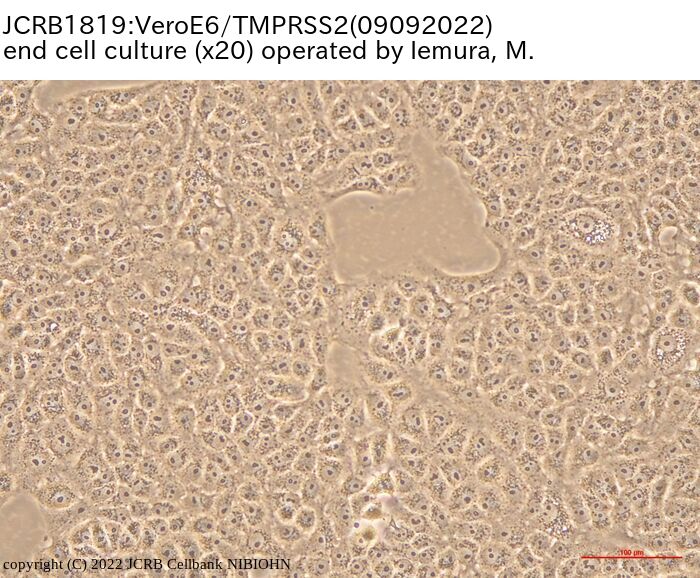
VeroE6 cells expressing the transmembrane serine protease TMPRSS2. |
Other Name |
|
| Animal |
monkey, African green |
Strain |
|
| Genus |
Chlorocebus |
Species |
sabaeus |
| Sex |
F |
Age |
|
| Identity |
|
Tissue for Primary Cancer |
|
| Case history |
|
Metastasis |
|
| Tissue Metastasized |
|
Genetics |
Cells were transfected with pAP3neo encoding human TMPRSS2 (plasmid vector) |
| Life Span |
infinite |
Crisis PDL |
|
| Morphology |
epithelial-like |
Character |
VeroE6 cells expressing the transmembrane serine protease TMPRSS2. |
| Classify |
transformed |
Established by |
Maino Tahara |
| Registered by |
Makoto Takeda |
Regulation for Distribution |
|
| Comment |
The female origin was determined by karyotype analysis with FISH in Vero. The scientific name of African green monkey was described as Cercopithecus aethiops at Vero cell establishment. |
Year |
|
| Medium |
10% FBS-DMEM, Penicillin (100 unit/mL), Streptomycin (100 ug/mL), Geneticin (G418)(1mg/ml) (at the establishment). Current medium for propagation: DMEM (low glucose) with 10% FBS and 1 mg/mL G418. |
Methods for Passages |
Treatment with trypsin-EDTA |
| Cell Number on Passage |
|
Race |
|
| CO2 Conc. |
5% |
Tissue Sampling |
kidney |
| Tissue Type |
|
| Reference |
| Pubmed id:32165541 | Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells.
Matsuyama S,Nao N,Shirato K,Kawase M,Saito S,Takayama I,Nagata N,Sekizuka T,Katoh H,Kato F,Sakata M,Tahara M,Kutsuna S,Ohmagari N,Kuroda M,Suzuki T,Kageyama T,Takeda M
Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7001-7003
|
|---|
| Pubmed id:31013314 | Consensus and variations in cell line specificity among human metapneumovirus strains.
Nao N,Sato K,Yamagishi J,Tahara M,Nakatsu Y,Seki F,Katoh H,Ohnuma A,Shirogane Y,Hayashi M,Suzuki T,Kikuta H,Nishimura H,Takeda M
PLoS One. 2019;14(4):e0215822
|
|---|