JCRB1371 GS-3A4-HepG2
Cell information
Cell type:genetically-modified cells (View Pricing Information)
| JCRB No. | JCRB1371 | Cell Name | GS-3A4-HepG2 |
|---|---|---|---|
| Profile | Human glutamine synthetase gene and drug-metabolizing CYP3A4 gene introduced Hep G2 cells. | Other Name | |
| Animal | human | Strain | |
| Genus | Homo | Species | sapiens |
| Sex | M | Age | 15 |
| Identity | available | Tissue for Primary Cancer | liver |
| Case history | Liver cancer | Metastasis | |
| Tissue Metastasized | Genetics | human glutamine synthetase and CYP3A4 introduced | |
| Life Span | infinite | Crisis PDL | |
| Morphology | epithelial-like | Character | |
| Classify | transformed | Established by | Omasa, T. |
| Registered by | Omasa, T. | Regulation for Distribution | First submission should be under joint authorship with the developer. |
| Comment | Year | 2009 | |
| Medium | RDF medium containing glucose (2.58 g/L), glutamine (333 mg/L), and 10% dialyzed fetal bovine serum. RDF medium is a 2:1:1 mixture of RPMI1640, DMEM, Ham's F12 without glucose and glutamine. | Methods for Passages | Cells harvested after treatment with 0.25% trypsin. |
| Cell Number on Passage | Race | ||
| CO2 Conc. | 5% | Tissue Sampling | Liver |
| Tissue Type | differentiated liver carcinoma |
| Detection of virus genome fragment by Real-time PCR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected DNA Virus | tested | Detected RNA Virus | tested | ||||||
| CMV | - | parvoB19 | - | HCV | - | HTLV-1 | - | ||
| EBV | - | HBV | - | HIV-1 | - | HTLV-2 | - | ||
| HHV6 | - | HTLV-1 | - | HIV-2 | - | HAV | - | ||
| HHV7 | - | HTLV-2 | - |
-/negative. +/positive. nt/not tested. (positive (+) does not immediately mean the production of infectious viral particles.) |
|||||
| BKV | - | HIV-1 | - | ||||||
| JCV | - | HIV-2 | - | ||||||
| ADV | - | HPV18 | - | ||||||
| Notes | |||||||||
| Reference | |
|---|---|
| Pubmed id:18846481 | Human CYP3A4-introduced HepG2 cells: in vitro screening system of new chemicals for the evaluation of CYP3A4-inhibiting activity. Araki N,Tsuruoka S,Wang N,Hasegawa G,Yanagihara H,Ando H,Omasa T,Enosawa S,Nagai H,Fujimura A Xenobiotica. 2008 Nov;38(11):1355-64 |
| Pubmed id:17300000 | Double-compartment cell culture apparatus: construction and biochemical evaluation for bioartificial liver support. Takahashi M,Sakurai M,Enosawa S,Omasa T,Tsuruoka S,Matsumura T Cell Transplant. 2006;15(10):945-52 |
| Pubmed id:16048486 | The bioreactor with CYP3A4- and glutamine synthetase-introduced HepG2 cells: treatment of hepatic failure dog with diazepam overdosage. Wang N,Tsuruoka S,Yamamoto H,Enosawa S,Omasa T,Sata N,Matsumura T,Nagai H,Fujimura A Artif Organs. 2005 Aug;29(8):681-4 |
| Pubmed id:15903254 | Construction and evaluation of drug-metabolizing cell line for bioartificial liver support system. Omasa T,Kim K,Hiramatsu S,Katakura Y,Kishimoto M,Enosawa S,Ohtake H Biotechnol Prog. 2005 Jan-Feb;21(1):161-7 |
| Images |
|---|
 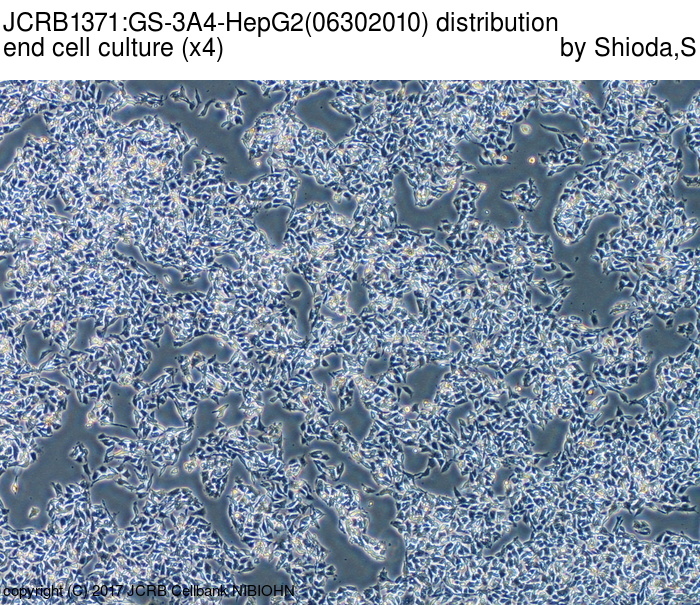 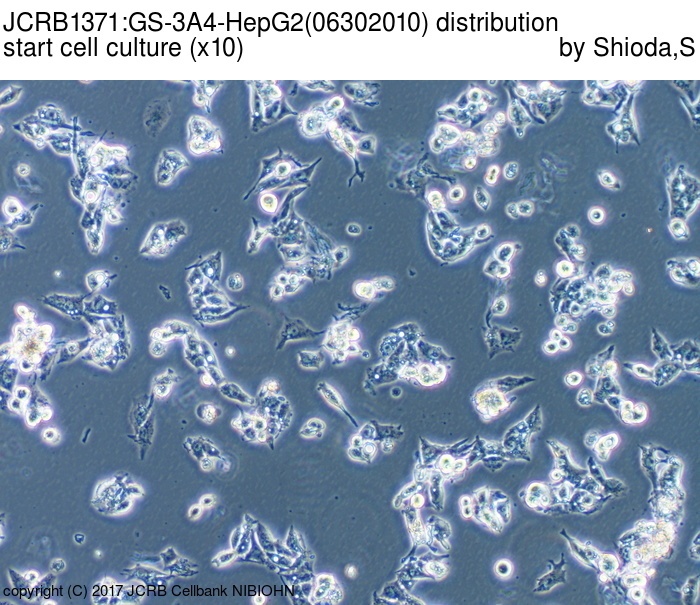 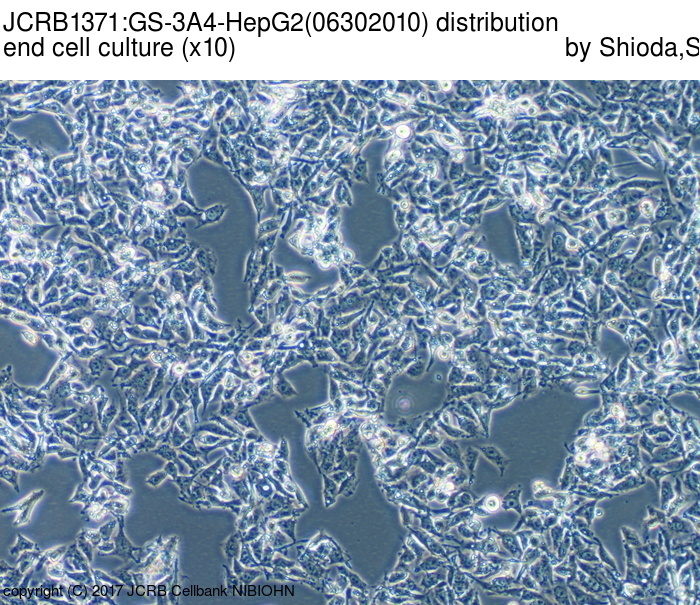 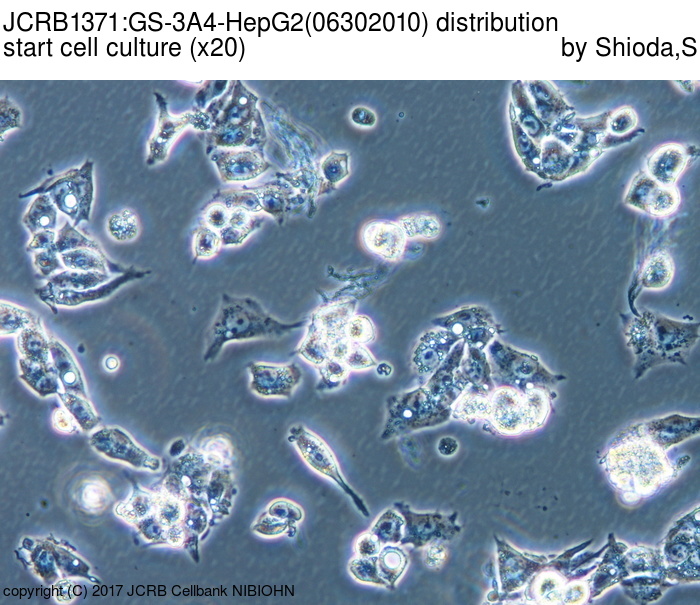   |
LOT Information
| Cell No. | JCRB1371 | Cell Name | GS-3A4-HepG2 |
|---|---|---|---|
| LOT No. | 06302010 | Lot Specification | distribution |
| Medium | RPMI1640(Invitrogen11875) :DMEM /F12(1/1) medium with10% dialized fetal bovine serum(Hyclone ARG26916) | Temperature | 37 C |
| Cell Density at Seeding | 3.7-5.0x10^4 cells/sq.cm | Methods for Passages | Cells were harvested after treatment with 0.25%trypsin(GIBCO) |
| Doubling Time | NT | Cell Number in Vial (cells/1ml) | 5.2x10^6 |
| Viability at cell freezing (%) | 99.0 | Antibiotics Used | free |
| Passage Number | p4* | PDL | NT |
| Sterility: MYCOPLASMA | - | Sterility: BACTERIA | - |
| Sterility: FUNGI | - | Isozyme Analysis | NT |
| Chromosome Mode | NT | Chromosome Information | NT |
| Surface Antigen | NT | DNA Profile (STR) | D5S818:11,12 D13S317:9,13 D7S820:10 D16S539:12 VWA:17 TH01:9 AM:X,Y TPOX:8,9 CSF1PO:10,11 |
| Adhesion | Yes | Exoteric Gene | NT |
| Medium for Freezing | Cell Banker BLC-1(Nihon Zenyaku Industries) | CO2 Conc. | 5 % |
| Viability immediately after thawing (%) | Additional information |
| Images |
|---|
       |