JCRB0435 JHH-4
細胞情報
Important Notice(s)On the agreement for distribution of JHH series cell lines, NOZ and OZ
細胞種類:一般細胞 (細胞分譲手数料はこちら)
| 細胞番号(JCRB) | JCRB0435 | 細胞名 | JHH-4 |
|---|---|---|---|
| 生物種(日本語) | ヒト | 組織名(日本語) | 肝臓, 胆嚢 |
| コメント(日本語) | 肝細胞がん | プロフィール | Human hepatocellular carcinoma cell line. |
| 別名 | 動物名 | human | |
| 系統名 | 学名・属名 | Homo | |
| 学名・種小名 | sapiens | 性別 | M |
| 年齢・月齢 | 51 | 細胞識別情報 | available |
| (癌)原発組織名 | liver, gallbladder | 病歴情報 | hepatocellular carcinoma |
| 転移の有無(Y/N) | (癌)転移組織名 | ||
| 遺伝的性質 | HBV integration is not observed. | 細胞寿命 | infinite |
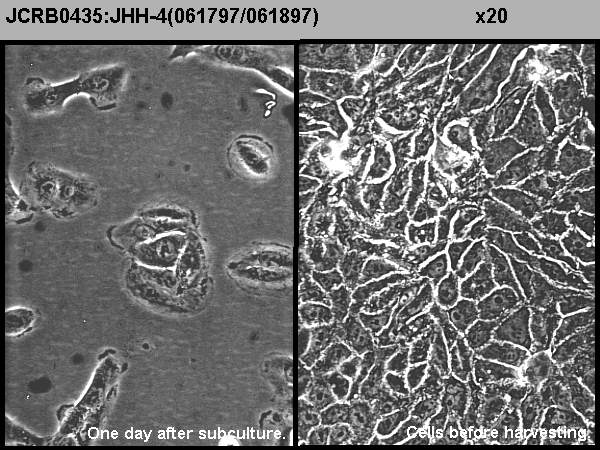
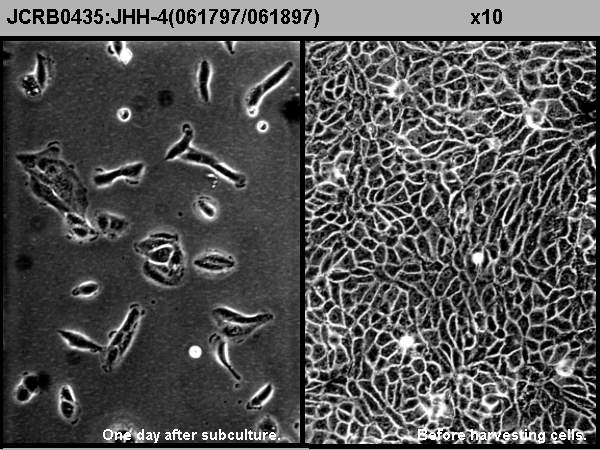
| クライシスPDL | 形態 | epithelial-like | |
| 一般性状 | Alpha-phetoprotein and albumin-producing cell line. HBs antigen-negative. HCV was not detected by an RT-PCR method tested in the HSRRB. | 細胞分類 | tumor |
| 細胞樹立者名 | Hasumura,S. et al. | 細胞寄託者 | Nagamori,S. |
| 分譲時制限 | Restricted: Cell's developer's approval is required | コメント | Cell Bank will handle to get the approval but the depositor may contact to the requested person directly. |
| 入手年 | 1988 | 培養培地 | Eagle's minimal essential medium with 10% fetal calf serum. |
| 継代方法 | Cells are harvested after the treatment with 0.25% trypsin and 0.02% EDTA at room temp. for 5 min. | 継代時細胞数 | 1-2x10^4 cells/sq.cm. |
| 人種 | Japanese | 炭酸ガス濃度 | 5 % |
| 採取組織名 | liver | 組織型 |
| ウイルスDNA・RNA検出検査 (Detection of virus genome fragment by Real-time PCR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ウイルスDNA 検出検査 |
tested | ウイルスRNA 検出検査 |
tested | ||||||
| CMV | - | parvoB19 | - | HCV | - | HTLV-1 | - | ||
| EBV | - | HBV | - | HIV-1 | - | HTLV-2 | - | ||
| HHV6 | - | HTLV-1 | - | HIV-2 | - | HAV | - | ||
| HHV7 | - | HTLV-2 | - |
-/negative. +/positive. nt/not tested. (positive (+) does not immediately mean the production of infectious viral particles.) |
|||||
| BKV | - | HIV-1 | - | ||||||
| JCV | - | HIV-2 | - | ||||||
| ADV | - | HPV18 | - | ||||||
| Notes | |||||||||
| Reference | |
|---|---|
| Pubmed id:2856444 | [Establishment and characterization of a human hepatocellular carcinoma cell line JHH-4]. Hasumura S,Sujino H,Nagamori S,Kameda H Hum Cell. 1988 Mar;1(1):98-100 |
| Research results by users. Click! | |
|---|---|
| Pubmed id:31324698 |
The 2-Hydroxyiminostilbene Metabolite of Carbamazepine or the Supernatant from Incubation of Hepatocytes with Carbamazepine Activates Inflammasomes: Implications for Carbamazepine-Induced Hypersensitivity Reactions. Kato R,Ijiri Y,Hayashi T,Uetrecht J Drug Metab Dispos. 2019 Oct;47(10):1093-1096 |
| Pubmed id:29087505 |
Editor's Highlight: An Impaired Immune Tolerance Animal Model Distinguishes the Potential of Troglitazone/Pioglitazone and Tolcapone/Entacapone to Cause IDILI. Mak A,Kato R,Weston K,Hayes A,Uetrecht J Toxicol Sci. 2018 Feb 1;161(2):412-420 |
| Pubmed id:28081667 |
Contributions of caspase-8 and -9 to liver injury from CYP2E1-produced metabolites of halogenated hydrocarbons. Ijiri Y,Kato R,Sadamatsu M,Takano M,Yasuda Y,Tanaka F,Oishi C,Imano H,Okada Y,Tanaka K,Hayashi T Xenobiotica. 2018 Jan;48(1):60-72 |
| Pubmed id:28525267 |
Supernatant from Hepatocyte Cultures with Drugs That Cause Idiosyncratic Liver Injury Activates Macrophage Inflammasomes. Kato R,Uetrecht J Chem Res Toxicol. 2017 Jun 19;30(6):1327-1332 |
| Pubmed id:27183710 |
Effect of hypoxia on UDP-glucuronosyl transferase mRNA expression in human hepatocarcinoma functional liver celL4 cell line. Kato R,Matsura A,Kamiya R,Oishi C,Kagawa Y,Tanaka F,Matsumoto N,Ijiri Y,Hayashi T Pharmazie. 2016 Mar;71(3):152-3 |
| Pubmed id:24599999 |
Novel permissive cell lines for complete propagation of hepatitis C virus. Shiokawa M,Fukuhara T,Ono C,Yamamoto S,Okamoto T,Watanabe N,Wakita T,Matsuura Y J Virol. 2014 May;88(10):5578-94 |
| Pubmed id:20044606 |
Infrequent amplification of JUN in hepatocellular carcinoma. Endo M,Yasui K,Nakajima T,Gen Y,Tsuji K,Dohi O,Zen K,Mitsuyoshi H,Minami M,Itoh Y,Taniwaki M,Tanaka S,Arii S,Okanoue T,Yoshikawa T Anticancer Res. 2009 Dec;29(12):4989-94 |
| Pubmed id:18248513 |
Chemosensitivity determinants of irinotecan hydrochloride in hepatocellular carcinoma cell lines. Takahata T,Ookawa K,Suto K,Tanaka M,Yano H,Nakashima O,Kojiro M,Tamura Y,Tateishi T,Sakata Y,Fukuda S Basic Clin Pharmacol Toxicol. 2008 Apr;102(4):399-407 |
| Pubmed id:17549407 |
Activation of a system A amino acid transporter, ATA1/SLC38A1, in human hepatocellular carcinoma and preneoplastic liver tissues. Kondoh N,Imazeki N,Arai M,Hada A,Hatsuse K,Matsuo H,Matsubara O,Ohkura S,Yamamoto M Int J Oncol. 2007 Jul;31(1):81-7 |
| Pubmed id:16041541 |
Interferon-alpha/beta upregulate IL-15 expression in vitro and in vivo: analysis in human hepatocellular carcinoma cell lines and in chronic hepatitis C patients during interferon-alpha/beta treatment. Yamaji K,Nabeshima S,Murata M,Chong Y,Furusyo N,Ikematsu H,Hayashi J Cancer Immunol Immunother. 2006 Apr;55(4):394-403 |
| Pubmed id:16522372 |
A comparison of the antitumor effects of interferon-alpha and beta on human hepatocellular carcinoma cell lines. Murata M,Nabeshima S,Kikuchi K,Yamaji K,Furusyo N,Hayashi J Cytokine. 2006 Feb 7;33(3):121-8 |
| Pubmed id:15349899 |
Proteomic signature corresponding to alpha fetoprotein expression in liver cancer cells. Yokoo H,Kondo T,Fujii K,Yamada T,Todo S,Hirohashi S Hepatology. 2004 Sep;40(3):609-17 |
| Pubmed id:10766424 |
Involvement of insulin-like growth factor binding protein-3 in the retinoic acid receptor-alpha-mediated inhibition of hepatocellular carcinoma cell proliferation. Murakami K,Matsuura T,Hasumura S,Nagamori S,Yamada Y,Saiki I Cancer Lett. 2000 Apr 3;151(1):63-70 |
| Pubmed id:9446792 |
Cyclin E overexpression responsible for growth of human hepatic tumors with p21WAF1/CIP1/SDI1. Tsuji T,Miyazaki M,Fushimi K,Mihara K,Inoue Y,Ohashi R,Ohtsubo M,Hamazaki K,Furusako S,Namba M Biochem Biophys Res Commun. 1998 Jan 14;242(2):317-21 |
| Pubmed id:8835345 |
Persistence of hepatitis C virus RNA in established human hepatocellular carcinoma cell lines. Tsuboi S,Nagamori S,Miyazaki M,Mihara K,Fukaya K,Teruya K,Kosaka T,Tsuji T,Namba M J Med Virol. 1996 Feb;48(2):133-40 |
| Pubmed id:1315167 |
Induction of allogeneic tumour- and lymphokine-activated lymphocytes against hepatocellular carcinoma. Yasumura S,Higuchi K,Hioki O,Okada K,Tsukishiro T,Tsuchida T,Miyagiwa M,Nambu S,Yasuyama T,Inoue K J Gastroenterol Hepatol. 1992 Mar-Apr;7(2):136-41 |
| Pubmed id:1651985 |
Preferential expression of the large hepatitis B virus surface antigen gene by an adenovirus-hepatitis B virus recombinant. Yuasa T,Kajino K,Saito I,Miyamura T J Gen Virol. 1991 Aug;72 ( Pt 8)():1927-34 |
| Pubmed id:1701409 |
Integration of hepatitis B virus DNA into cells of six established human hepatocellular carcinoma cell lines. Fujise K,Nagamori S,Hasumura S,Homma S,Sujino H,Matsuura T,Shimizu K,Niiya M,Kameda H,Fujita K Hepatogastroenterology. 1990 Oct;37(5):457-60 |
| Pubmed id:2543327 |
[Combination therapy of hyperthermia and other methods in liver and bile tract cancers--evaluation of these methods using cancer cell lines in vitro]. Hasumura S,Nagamori S,Fujise K,Homma S,Sujino H,Matsuura T,Shimizu K,Niiya M,Kameda H Gan To Kagaku Ryoho. 1989 Apr;16(4 Pt 2-3):1905-12 |
| Pubmed id:2856500 |
[Effects of TNF on human hepatocellular carcinoma cell lines and their modification by hyperthermia]. Hasumura S,Nagamori S,Fujise K,Homma S,Sujino H,Matsuura T,Shimizu K,Niiya M,Kameda H,Satomi N Hum Cell. 1988 Jun;1(2):238-44 |
| Pubmed id:2856444 |
[Establishment and characterization of a human hepatocellular carcinoma cell line JHH-4]. Hasumura S,Sujino H,Nagamori S,Kameda H Hum Cell. 1988 Mar;1(1):98-100 |
| Pubmed id:3428672 |
An epidemic of pseudomembranous colitis: importance of person to person spread. Nolan NP,Kelly CP,Humphreys JF,Cooney C,O'Connor R,Walsh TN,Weir DG,O'Briain DS Gut. 1987 Nov;28(11):1467-73 |
| Pubmed id:none |
THE MORPHOLOGICAL STUDY OF HUMAN CULTURED HEPATOMA CELLS BY A NEW PLASMA POLYMERIZATION REPLICA METHOD AND TEM Hajime SUJINO Jikeikai Medical Journal 33(4), 379-400 (1986) |
| Pubmed id:none |
ヒト肝癌細胞株に対するTumor necrosis factorの影響 蓮村哲 永森静志 藤瀬清隆 本間定 筋野甫 松浦知和 清水恵一郎 新谷稔 里見信子 医学のあゆみ 143(8), 655-656 (1987) |
| Pubmed id:none |
Fluorochromeを用いたchromatin DNAのcolorimetric assay法による単層培養細胞増殖動態の検討 松浦知和 永森静志 藤瀬清隆 蓮村哲 本間定 筋野甫 清水恵一郎 新谷稔 亀田治男 医学のあゆみ 142(13), 957-958 (1987) |
| Pubmed id:none |
樹立ヒト肝癌株細胞中のB型肝炎ウイルスゲノム 藤瀬 清隆, 藤多 和信, 永森 静志, 蓮村 哲, 本間 定, 筋野 甫, 松浦 知和, 清水 恵一郎, 新谷 稔, 大野 典也, 亀田 治男 肝臓 29(5), 697-698 (1988) |
| Images |
|---|
        |
| Movies |
|---|

|
LOT Information
| 細胞番号 | JCRB0435 | 細胞名 | |
|---|---|---|---|
| 培養ロット番号 | 04142005 | 培養種別 | distribution |
| 培地 | Eagle's minimal essential medium with 10% fetal bovine serum (FBS lot; GIBCO 4218333S) | 培養温度 | 37 C |
| 継代時細胞数(濃度) | 5 - 15 x 10^4 cells/ml | 継代方法 | 0.25% trypsin and 0.02% EDTA at room temperature for 5 min. |
| 増殖速度 | approx. 40.5 hrs | 凍結時生細胞濃度 | 2.3 x 10^6 |
| 凍結時生細胞率 | 97.1 | 使用抗生物質 | free |
| 継代数 | P17 | PDL数(プライマリ) | |
| マイコプラズマ検出 | - | 細菌汚染検出 | - |
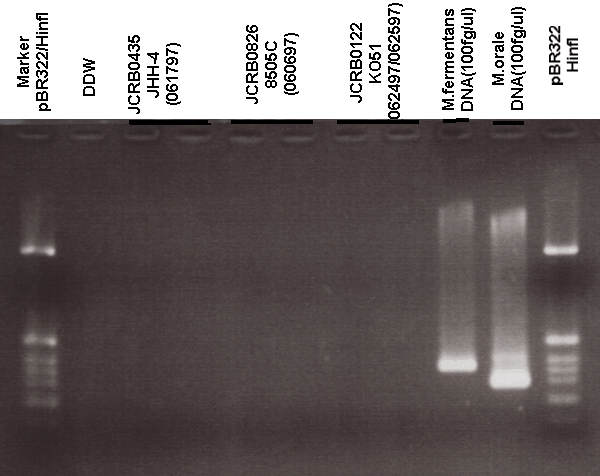
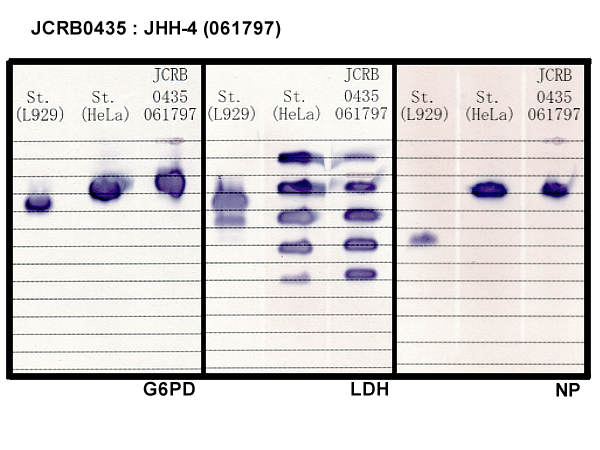
| 真菌汚染検出 | - | アイソザイム検査・動物名 | Confirmed as human by NP, G6PD (type B), MD. |
| 染色体モード | 染色体情報 | ||
| 表面抗原 | DNA Profile (STR) | ||
| 接着性 | Yes | 導入外部遺伝子 | |
| 凍結培地 | 10% DMSO, 20% FBS - Eagle's MEM | 炭酸ガス濃度 | 5% |
| 解凍後生細胞率 | 追加情報 |
| 細胞番号 | JCRB0435 | 細胞名 | JHH-4 |
|---|---|---|---|
| 培養ロット番号 | 061897 | 培養種別 | distribution |
| 培地 | Eagle's minimal essential medium with 10% fetal calf serum (Mitsubishi PVF01). | 培養温度 | 37 C |
| 継代時細胞数(濃度) | 1-2x10^6 cells/sq.cm. | 継代方法 | Cells are harvested after the treatment with 0.25% trypsin and 0.02% EDTA at room temp. for 5 min. |
| 増殖速度 | NT | 凍結時生細胞濃度 | 3.0x10^6 |
| 凍結時生細胞率 | 92.0 | 使用抗生物質 | free |
| 継代数 | P13 | PDL数(プライマリ) | NT |
| マイコプラズマ検出 | - | 細菌汚染検出 | - |
| 真菌汚染検出 | - | アイソザイム検査・動物名 | NT |
| 染色体モード | NT | 染色体情報 | NT |
| 表面抗原 | NT | DNA Profile (STR) | |
| 接着性 | Yes | 導入外部遺伝子 | NT |
| 凍結培地 | Culture mdium with 5% DMSO. | 炭酸ガス濃度 | |
| 解凍後生細胞率 | 追加情報 |
| Images |
|---|
    |
| 細胞番号 | JCRB0435 | 細胞名 | JHH-4 |
|---|---|---|---|
| 培養ロット番号 | 04142014 | 培養種別 | distribution |
| 培地 | Eagle's minimal essential medium with 10% fetal calf serum (FBS; GIBCO Cat. # 10091). | 培養温度 | 37 C |
| 継代時細胞数(濃度) | 4.7 - 9.0 x 10^4 cells/mL | 継代方法 | Cells were harvested after the treatment with 0.25% trypsin and 0.02% EDTA. |
| 増殖速度 | 凍結時生細胞濃度 | 2.9 x 10^6 | |
| 凍結時生細胞率 | 96.0 | 使用抗生物質 | free |
| 継代数 | P17 | PDL数(プライマリ) | |
| マイコプラズマ検出 | - | 細菌汚染検出 | - |
| 真菌汚染検出 | - | アイソザイム検査・動物名 | |
| 染色体モード | 染色体情報 | ||
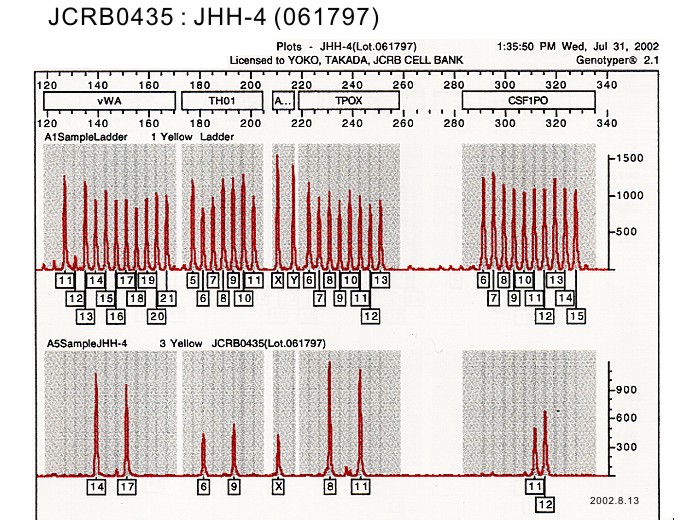
| 表面抗原 | DNA Profile (STR) | D5S818:9,13 D13S317:12 D7S820:10,12 D16S539:12,13 VWA:14,17 TH01:6,9 AM:X TPOX:8,11 CSF1PO:11,12 |
|
| 接着性 | Yes | 導入外部遺伝子 | |
| 凍結培地 | 10% DMSO, 20% FBS - EMEM | 炭酸ガス濃度 | 5% |
| 解凍後生細胞率 | 96.0 | 追加情報 | Cell Bank will handle to get the approval but the depositor may contact to the requester directly. |
| 細胞番号 | JCRB0435 | 細胞名 | JHH-4 |
|---|---|---|---|
| 培養ロット番号 | 01242022 | 培養種別 | distribution |
| 培地 | Eagle's minimum essential medium with 10% fetal bovine serum (FBS; Biowest Cat. # S18200-500) | 培養温度 | 37 C |
| 継代時細胞数(濃度) | 0.4 - 2.0 x 10^5 cells/mL | 継代方法 | Cells were harvested after treatment with 0.25% trypsin and 0.02% EDTA. |
| 増殖速度 | approx. 32 hrs. | 凍結時生細胞濃度 | 1.6 x 10^6 |
| 凍結時生細胞率 | 97 | 使用抗生物質 | free |
| 継代数 | P16 | PDL数(プライマリ) | |
| マイコプラズマ検出 | - | 細菌汚染検出 | - |
| 真菌汚染検出 | - | アイソザイム検査・動物名 | |
| 染色体モード | 染色体情報 | ||
| 表面抗原 | DNA Profile (STR) | ||
| 接着性 | Yes | 導入外部遺伝子 | |
| 凍結培地 | 10% DMSO, 20% FBS - EMEM | 炭酸ガス濃度 | 5% |
| 解凍後生細胞率 | 92 | 追加情報 |